At WBMP, our mission is to develop highly effective, more tumor-specific monoclonal antibody (mAb) therapies. We design potent antibodies that can directly induce a therapeutic effect, not requiring the use of toxic effector compounds, and which do not cross-react with healthy tissues. This approach allows for the development of safer, more effective, and more affordable treatment options for the diseases we target.
About Our Antibodies
Drug Discovery
Inspired by our research into, and discovery of, the mechanism of action of anti-HER2 antibodies, we generated a proprietary drug discovery platform to identify novel therapeutic targets on membrane receptors. We have successfully applied this approach to the development of a patented panel of first-in-class monoclonal antibodies (mAbs) to treat B-cell malignancies.
Lead Product
Non-Hodgkin Lymphoma (NHL) and Chronic Lymphocytic Leukemia (CLL) consist of a heterogeneous group of hematological diseases, 80% of which are B-cell in origin. Currently available therapies for these diseases are often not specific to B-cells, as they target proteins that are also found in non-cancerous cells, leading to off-target effects, including secondary cancers. While therapies that are more specific, wipe out all B-cells, including healthy cells, resulting in severe immune suppression and dysregulation.
Our class-specific antibodies target theIg component of the B-cell Receptor Complex (BCRC). The BCRC controls cell survival, metabolism, growth, proliferation, and differentiation programs in normal B-cells. However, in malignant B-cells, the BCRC is constitutively activated and is the driver of inappropriate and continuous survival, proliferation, and growth signals that play a central role in the pathogenesis of these cancers, making it a rational drug target. Targeting the BCRC has the potential to affect all intracellular downstream pathways, a mechanism of action that is not currently achieved by any existing therapy.Using our drug target discovery platform, we identified critical epitopes on membrane IgM (mIgM) of the BCRC, which we predicted would induce growth inhibition and apoptosis upon antibody binding. We have generated a panel of four mAbs (WBMP-1, WBMP-2, WBMP-3, and WBMP-4) spanning this targeted epitope.
Gold-labeled WBMP-4 binds to monomeric andclustered mIgM on a Burkittlymphoma cell.
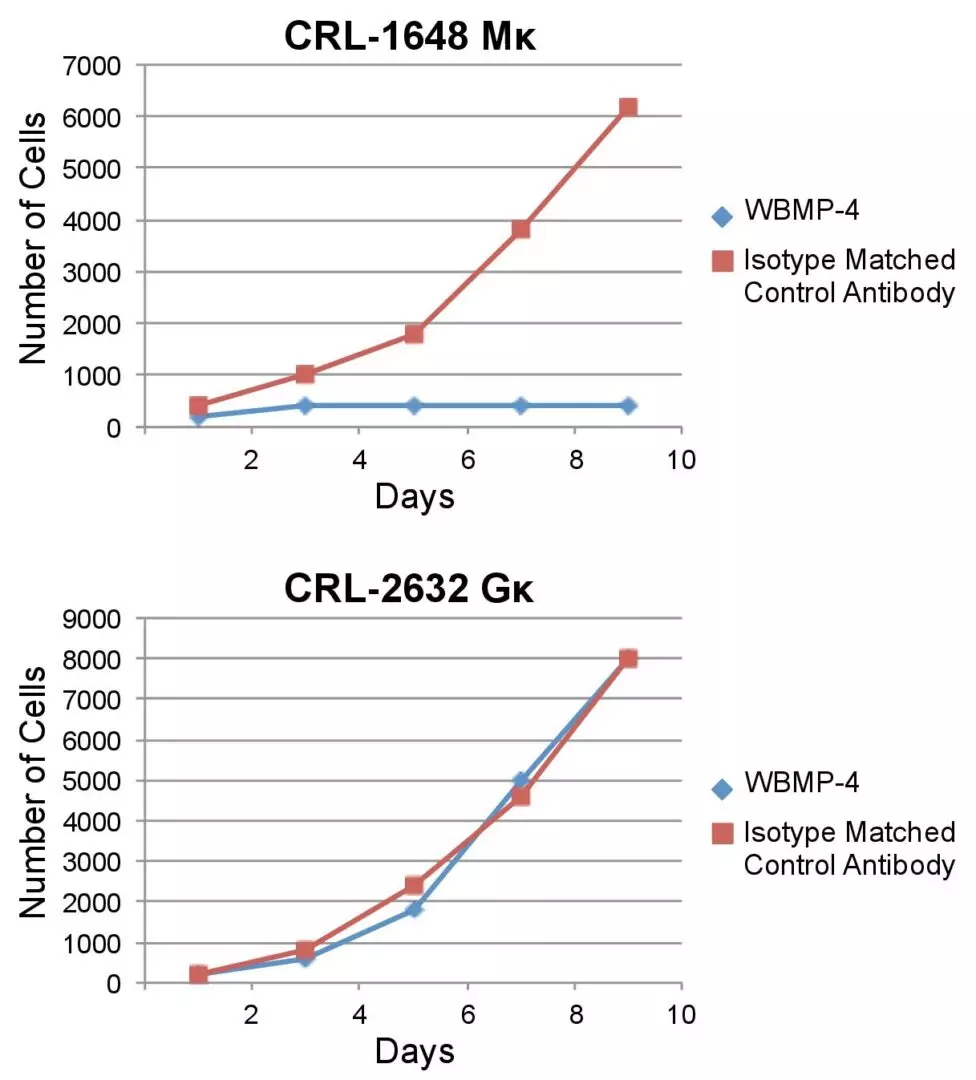
WBMP-4 binding induces growth inhibition and apoptosis in IgM-expressingBurkitt lymphoma cells (top) but not in IgG-expressing diffuse large B-cell lymphoma (bottom).
Proof of Principle
Our panel of anti-BCRC mAbs exhibit high specificity for lymphoma and leukemia cells, and WBMP-4 induces receptor internalization, growth inhibition, apoptosis, and manifests anti-stem-cell like activities in human lymphoma and leukemia cell lines.Based on its higher level of specificity, and potent direct anti-tumor effects demonstrated in vitro, we predict WBMP-4 will supplant current antibody targeting systems, small molecule drugs, and other pipeline products in NHL and CLL therapies.
We are currently finishing pre-clinical development of these anti-BCRC antibodies, while analyzing the specific effects of WBMP-4 on BCRC-controlled intracellular signaling pathways.
Pipeline
WBMP is working to develop mAbs targeting additional markers of B-cell, and other hematological malignancies, as well as driver receptors for colon, lung, and breast cancers.
